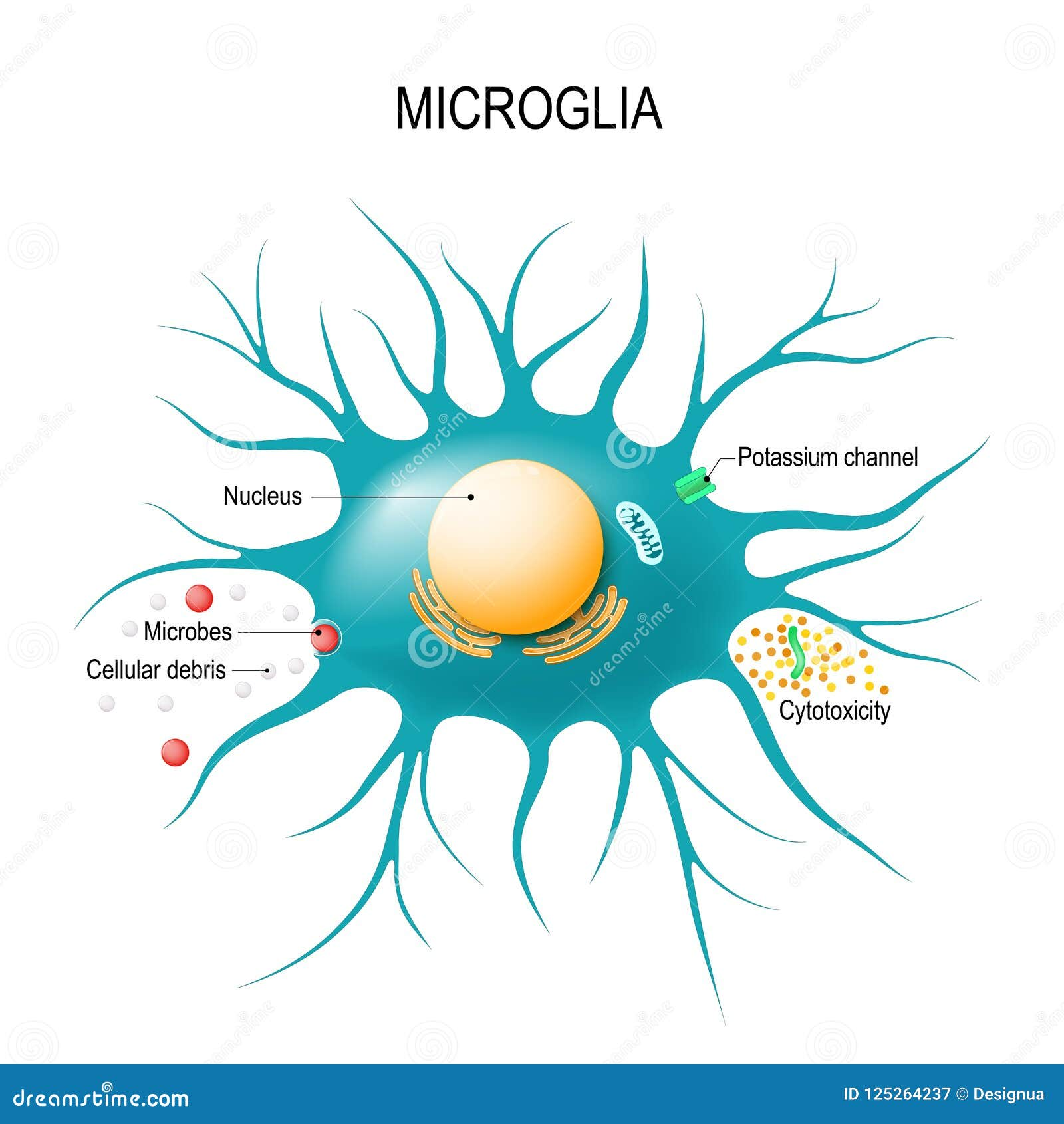
Microglial cells serve as the brain’s immune guardians, playing a critical role in maintaining neural health and functionality. These specialized cells are primarily responsible for monitoring the brain environment, clearing away cellular debris, and performing synaptic pruning, which is essential for the development of neuronal connections. Recent advancements in Alzheimer’s research have shed light on how dysfunctional microglial activity can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s disease. Dr. Beth Stevens, a prominent figure in this field, emphasizes that understanding microglia opens new avenues for innovative treatments and potential biomarkers for these devastating conditions. By focusing on the crucial interactions between microglial cells and neuronal networks, researchers aim to develop strategies that not only address Alzheimer’s but also enhance our overall understanding of brain health and its immune mechanisms.
Often described as the custodians of the central nervous system, microglial cells act as the brain’s first line of defense against injury and disease. They are essential for maintaining homeostasis within the brain, as they detect and respond to pathological changes while also engaging in processes like synaptic remodeling during development. This immune function is pivotal in the context of neurodegenerative disorders, including conditions such as Alzheimer’s disease, where altered microglial behavior can worsen synapse maintenance and brain connectivity. The work of researchers, particularly Beth Stevens, highlights the importance of these immune cells in understanding not just neurodegenerative diseases but also the fundamental mechanisms of brain health. As scientists delve deeper into the dynamic roles of these cells, the potential for breakthroughs in neurobiology and therapeutics continues to expand.
Understanding the Role of Microglial Cells in Neurodegenerative Diseases
Microglial cells serve as the brain’s primary immune defenders, constantly surveying the environment for signs of damage or disease. These cells are crucial for maintaining brain health by removing dead or damaged cells and facilitating synaptic pruning—an essential process that shapes neural circuitry. As highlighted by Beth Stevens’ research, abnormalities in microglial function can lead to excessive synaptic pruning, which is linked to various neurodegenerative diseases, including Alzheimer’s and Huntington’s disease. This underscores the importance of understanding microglial biology in the context of Alzheimer’s research, as it can provide deeper insights into disease mechanisms and potential therapeutic targets.
Stevens’ work illuminates the dual role of microglial cells in both safeguarding and potentially harming the brain. While they protect against disease, their involvement in neuroinflammation and altered synaptic pruning can contribute to the progression of neurodegenerative disorders. This paradox highlights the complexity of the brain’s immune system, as it must balance effective defense with the preservation of neural networks. As researchers continue to explore the intricacies of microglial function, there is hope that this knowledge can pave the way for innovative treatments for Alzheimer’s and other related conditions.
The Impact of Synaptic Pruning in Alzheimer’s Disease
Synaptic pruning, the process by which unnecessary neural connections are eliminated, is vital for the efficient functioning of the brain. In a healthy brain, microglial cells play an essential role in this process, ensuring that only the most useful synapses are retained. However, in conditions like Alzheimer’s disease, this process can become dysregulated. As underscored by the findings from Stevens’ lab, abnormal synaptic pruning may lead to cognitive impairments and contribute to the neurodegenerative cascade seen in Alzheimer’s patients. Understanding how synaptic pruning goes awry in Alzheimer’s is crucial for developing new biomarkers and therapeutic strategies.
The study of synaptic pruning is also essential for recognizing how early interventions might slow the progression of neurodegenerative diseases. Early detection, coupled with interventions targeting microglial activity, could help modulate synaptic pruning processes before significant cognitive decline occurs. This approach could revolutionize Alzheimer’s research by not only focusing on treatment but also on prevention, thereby potentially improving the quality of life for millions affected by the disease.
Beth Stevens: A Pioneer in Microglial Research
Beth Stevens has emerged as a leading figure in the field of neuroscience, particularly through her groundbreaking research on microglial cells. Her work has significantly altered the perception of the brain’s immune system, emphasizing that microglia are not merely passive participants but active modulators of synaptic health. Stevens’ blending of curiosity-driven research with cutting-edge techniques has led to a deeper understanding of how microglia contribute to synapse formation and removal, which has profound implications for Alzheimer’s and other neurodegenerative diseases.
Her contributions have not gone unnoticed; in 2015, Stevens received the prestigious MacArthur Fellowship, reinforcing her status as a thought leader in the study of microglia. Her focus on the basic science of microglial function illustrates the importance of foundational research in uncovering the complexities of neurodegenerative disorders. By drawing connections between cellular mechanisms and disease outcomes, Stevens’ work exemplifies the transformative potential of scientific inquiry in addressing pressing health issues like Alzheimer’s.
The Intersection of Neuroinflammation and Neurodegeneration
The relationship between neuroinflammation and neurodegeneration is a critical area of study in understanding diseases such as Alzheimer’s. Microglial cells are central to this relationship, as they can mediate both protective and harmful inflammatory responses. Chronic activation of microglia often leads to neuroinflammation, which has been implicated in the degeneration of neuronal cells and exacerbation of symptoms in Alzheimer’s disease. By investigating these mechanisms, researchers can discover ways to modulate microglial activity, potentially mitigating the impact of neuroinflammation on neurodegeneration.
As scientists continue to elucidate the roles of different inflammatory pathways, it becomes imperative to develop therapies that can target these specific pathways effectively. The insights gained from investigating neuroinflammation’s role in diseases like Alzheimer’s can significantly influence treatment strategies. Research pioneered by scientists like Beth Stevens could lead to therapeutic interventions that not only address symptoms but also tackle the underlying inflammatory processes contributing to neuronal damage.
Exploring New Biomarkers for Alzheimer’s Disease
The quest for reliable biomarkers in Alzheimer’s disease has gained momentum as researchers strive to improve diagnostic capabilities and treatment approaches. Stevens’ lab has made strides in identifying potential biomarkers linked to abnormal microglial function and synaptic pruning. These discoveries are pivotal, considering the early detection and intervention efforts being pursued in Alzheimer’s research. Biomarkers that reflect microglial activity could enable clinicians to diagnose Alzheimer’s more accurately and swiftly, providing a roadmap for personalized therapeutic strategies.
An effective biomarker could revolutionize Alzheimer’s treatment by allowing for earlier and more targeted interventions. By correlating microglial activity with clinical outcomes, the research conducted by Stevens and her team may lead to breakthroughs in understanding disease progression. Ultimately, the identification of such biomarkers will be instrumental in developing therapeutic approaches aimed at mitigating the effects of Alzheimer’s and enhancing the quality of life for those affected.
The Future of Neurodegenerative Disease Treatment
Advancements in scientific research are rapidly transforming our understanding of neurodegenerative diseases, particularly those like Alzheimer’s. With the ongoing exploration of microglial biology, great strides are being made toward innovative treatments that target the fundamental mechanisms of these diseases. The work of researchers like Beth Stevens is not only paving the way for potential new therapies but also igniting a paradigm shift in how we approach neurodegeneration, taking into consideration the brain’s immune responses.
By focusing on the interactions between microglia, synapses, and disease processes, there is hope for the development of interventions that can improve neuronal health and functioning. Future treatments may involve modulating microglial activity to restore balance in the immune response while preserving synaptic integrity. As research progresses, the insights gained will enhance our understanding of Alzheimer’s pathology and contribute to a future where these once-uncontrollable diseases may become manageable.
The Importance of Curiosity-Driven Research in Neuroscience
Curiosity-driven research plays a fundamental role in advancing our understanding of complex biological systems, particularly in neuroscience. Beth Stevens’ journey exemplifies how pursuing foundational questions can lead to significant discoveries that impact how we comprehend diseases like Alzheimer’s. This type of research often leads to unforeseen breakthroughs, as it allows scientists to explore various aspects of a problem without being constrained by immediate applications. By fostering an environment where curiosity can thrive, the scientific community can continue to explore novel avenues in the brain’s immune response and neurodegenerative disease mechanisms.
Moreover, the support from federal funding bodies highlights the necessity of investing in curiosity-driven research. As Stevens points out, foundational studies yield vital insights that can later inform practical applications. Supporting such research initiatives is essential for nurturing the next generation of scientists who will explore the unknown and revolutionize how we approach diseases of the brain, such as Alzheimer’s and others.
Federal Funding and Its Role in Alzheimer’s Research
Federal funding has been instrumental in supporting transformative research in Alzheimer’s and neurodegenerative diseases. As noted by Beth Stevens, much of her early work was made possible through grants from the National Institutes of Health (NIH). This financial backing provides researchers with the resources they need to explore innovative ideas and conduct experiments that would otherwise be hindered by budget constraints. The role of federal funding in neuroscience research cannot be overstated, as it underpins the foundational work that fuels advances in our understanding of complex diseases.
As the landscape of Alzheimer’s research evolves, continued investment in federal funding is essential to sustain progress. Funding enables interdisciplinary collaborations, fosters the exploration of new technologies, and supports long-term projects that delve into the intricacies of diseases affecting millions. By maintaining a robust investment in Alzheimer’s research, we not only honor the scientific curiosity that drives these fields but also work towards tangible improvements in treatment and care for those affected by neurodegenerative disorders.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are integral to Alzheimer’s research as they constitute the brain’s immune system. They monitor brain health, remove dead or damaged neurons, and participate in synaptic pruning. However, in Alzheimer’s disease, abnormal activity of microglial cells can contribute to neurodegenerative processes, making them a critical focus for researchers like Beth Stevens.
How do microglial cells contribute to neurodegenerative diseases?
Microglial cells contribute to neurodegenerative diseases by acting as the brain’s immune response. They can become overactive and lead to excessive synaptic pruning, a phenomenon linked to conditions such as Alzheimer’s and Huntington’s disease. The research led by Beth Stevens emphasizes the importance of understanding these mechanisms to develop potential treatments.
What is synaptic pruning and how is it related to microglial cells?
Synaptic pruning is a process in which microglial cells selectively eliminate unnecessary synapses in the brain. This process is essential for normal brain development but can become dysregulated in neurodegenerative diseases like Alzheimer’s, highlighting the tenacity of microglia in maintaining brain integrity and the emerging target for therapeutic strategies.
Why are microglial cells considered essential to the brain immune system?
Microglial cells are essential to the brain’s immune system because they constantly survey the brain environment for pathogens, debris, and signs of injury. Their ability to phagocytize dead cells and manage inflammation is crucial for maintaining brain health and preventing neurodegenerative diseases, as underscored by research from Beth Stevens.
What new discoveries about microglial cells have come from Beth Stevens’ lab?
Beth Stevens’ lab has uncovered important insights regarding the dual role of microglial cells in brain development and neurodegenerative diseases. They have found that while microglia are vital for healthy synaptic pruning, their abnormal activity can lead to conditions like Alzheimer’s disease, thereby revealing critical biomarkers for early detection and possible treatment interventions.
How are researchers using microglial cells to advance treatments for Alzheimer’s?
Researchers are leveraging the understanding of microglial cells to develop new biomarkers and interventions for Alzheimer’s disease. By studying how these immune cells interact with synapses and their role in synaptic pruning, scientists, including Beth Stevens, aim to create therapeutic strategies that could alter the disease course and improve patient outcomes.
What impact do microglial cells have on synaptic function and neural circuits?
Microglial cells play a pivotal role in modulating synaptic function and shaping neural circuits. Through synaptic pruning, they ensure the elimination of weak or redundant connections, which is vital for efficient neural communication. Dysregulation of this process by microglial cells is associated with neurodegenerative diseases, emphasizing their importance in both health and disease.
What are the implications of abnormal microglial activity in neurodegenerative diseases?
Abnormal microglial activity can lead to excessive synaptic pruning and chronic inflammation, contributing to neurodegenerative diseases like Alzheimer’s. Understanding these mechanisms is crucial for developing targeted therapies that might counteract the detrimental effects of microglia, thereby opening new avenues for research and treatment as demonstrated by Beth Stevens’ work.
| Key Point | Details |
|---|---|
| Microglial Cells Function | Microglial cells are the brain’s immune system, responsible for scanning the brain for signs of illness or injury, removing dead or damaged cells, and pruning synapses. |
| Role in Neurodegenerative Diseases | Abnormal microglial pruning has been linked to Alzheimer’s disease, Huntington’s disease, and other disorders. |
| Research Support | Beth Stevens’ research is supported by federal funding, enabling critical advancements in understanding microglia. |
| Impact on Alzheimer’s Care | This research has the potential to improve care for the estimated 7 million Americans living with Alzheimer’s disease. |
| Curiosity-Driven Research | Foundational research can lead to unexpected practical applications and deepen understanding of diseases. |
Summary
Microglial cells play a crucial role in the brain’s immune response and are key players in neurodevelopment and neurodegeneration. Research led by Beth Stevens has shed light on their functions and the impact of abnormal pruning, providing insights into Alzheimer’s disease and paving the way for innovative treatments. Understanding the complexities of microglial cells can lead to significant advancements in how we approach neurodegenerative diseases, ultimately enhancing the quality of life for millions affected by such conditions.