Emerging research into TIM-3 therapy for Alzheimer’s is shedding light on a potential breakthrough in how we treat this devastating condition. TIM-3, a checkpoint molecule, plays a crucial role in regulating the function of brain immune cells known as microglia, which are responsible for clearing amyloid plaques associated with Alzheimer’s disease. In recent studies, eliminating TIM-3 expression allowed these microglia to successfully engage in plaque clearance, leading to improved memory and cognitive functions in mouse models. This innovative approach not only highlights TIM-3 therapy’s potential in Alzheimer’s disease treatment but also underscores the significance of uncovering novel mechanisms underlying the disease. As scientists delve deeper into the relationship between brain immune cells, plaque buildup, and cognitive improvement, the future of Alzheimer’s therapy may become brighter than ever.
Recent advancements in Alzheimer’s disease therapy have brought attention to a promising approach involving TIM-3 modulation. This immune checkpoint strategy, originally targeted for cancer treatment, seeks to enhance the activity of microglia—the brain’s resident immune cells. By inhibiting TIM-3, researchers aim to enable these cells to more effectively remove amyloid plaques, which are crucial contributors to Alzheimer’s pathology. Such therapeutic techniques could pave the way for significant cognitive enhancements and offer hope to those affected by this neurodegenerative condition. As we explore innovative avenues for Alzheimer’s management, the intersection of immunology and neurology is promising to reshape future treatment landscapes.
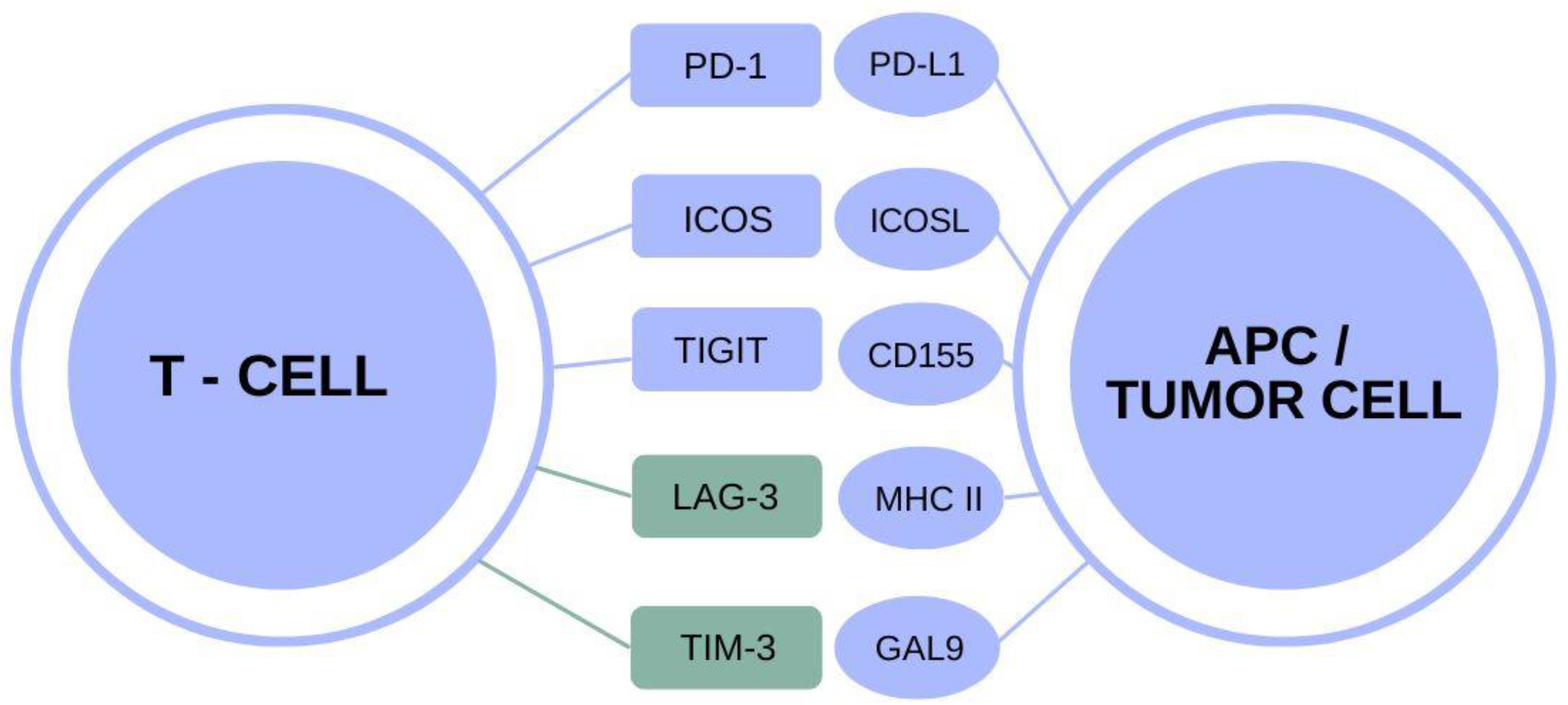
Understanding TIM-3 and Its Role in Alzheimer’s Disease
TIM-3, or T-cell immunoglobulin and mucin domain 3, is an immune checkpoint molecule that plays a pivotal role in regulating the immune response, particularly within the brain. Research has shown that TIM-3 expression is heightened in microglia, the brain’s immune cells, in the context of Alzheimer’s disease (AD). This overexpression prevents microglia from effectively clearing amyloid plaques, which are toxic aggregates that disrupt cognitive function. In patients genetically predisposed to late-onset Alzheimer’s, elevated TIM-3 levels can hinder the natural protective effects of microglia, leading to exacerbated plaque accumulation and memory decline.
The critical nature of TIM-3 in moderating immune responses signifies its potential as a target for therapeutic intervention. By inhibiting or blocking TIM-3, researchers aim to enable microglia to reclaim their functions of clearing harmful plaques from the brain. Understanding how TIM-3 operates could pave the way for developing innovative treatments that restore cognitive function and reverse some aspects of Alzheimer’s pathology.
Studies have indicated that when TIM-3 is deleted or inhibited, microglia become more aggressive in their attack on amyloid plaques, drastically improving, at least in animal models, cognitive behavior and memory recall. This discovery suggests a promising pathway for Alzheimer’s disease treatment strategies, where targeting TIM-3 could lead to significant cognitive improvement in individuals suffering from this debilitating disease. Given that most Alzheimer’s cases are late-onset, the implications of TIM-3 therapy could be monumental, as it addresses a significant underlying mechanism contributing to disease progression.
The Mechanism of Microglia in Alzheimer’s Disease
Microglia serve as the brain’s primary immune defenders, tasked with removing cellular debris and maintaining homeostasis. During the development of Alzheimer’s disease, however, microglial function is compromised. The expression of TIM-3 exacerbates this problem, leading microglia into a homeostatic state where they cease to clear amyloid beta plaques effectively. This dysfunction is particularly detrimental as the accumulation of these plaques is strongly correlated with cognitive decline and memory loss in Alzheimer’s patients. Consequently, understanding the dynamics of microglial activity in the presence of TIM-3 could unveil novel therapeutic strategies aimed at enhancing their plaque-clearing abilities.
Furthermore, the inhibition of plaque clearance by TIM-3 not only contributes to the toxic environment but also alters the fate of synapses essential for memory storage. The interplay between synaptic pruning and plaque accumulation highlights a crucial axis in Alzheimer’s pathology. By reactivating the ability of microglia to clear plaques, researchers could improve synaptic health, potentially leading to cognitive restoration and quality of life improvements in Alzheimer’s disease patients.
Current studies support the theory that re-establishing microglial function could alleviate symptoms associated with Alzheimer’s disease. Exploring avenues to manipulate TIM-3 expression, perhaps through pharmacological means, may rejuvenate the natural functions of these immune cells. Researchers are optimistic about the prospects of developing targeted therapies that can selectively disengage TIM-3 inhibition, thereby empowering microglia to perform their duties effectively and reducing the burden of amyloid plaques. Enhancing microglia function can represent a pivotal advance in Alzheimer’s disease treatment, with the aim of restoring cognitive capabilities.
Innovative Alzheimer’s Therapy: Targeting TIM-3
The development of TIM-3 therapy for Alzheimer’s disease represents a transformative strategy in addressing this neurodegenerative condition. Unlike conventional therapies that primarily focus on amyloid beta clearance without addressing the underlying immune dysfunction, TIM-3 therapy directly targets the blockade of inhibitory signals that impair microglial action. By utilizing anti-TIM-3 antibodies or small molecules, researchers aim to release microglia from their inhibitory state, thereby enhancing their efficacy in clearing harmful plaques and potentially improving cognitive functions in Alzheimer’s patients.
Transitioning TIM-3 as a therapeutic target signifies a shift in the paradigm of Alzheimer’s treatment, moving from merely symptomatic management towards addressing core pathological mechanisms. Given that traditional therapies have struggled to yield substantial cognitive improvement in clinical trials, the TIM-3 approach may offer much-needed hope for patients and caregivers alike, marking a significant progression toward finding effective treatments.
Initial research has shown promising results in animal models, where the inhibition of TIM-3 led to a noticeable decrease in plaque loads and improvements in cognitive behaviors. Future human trials with TIM-3 inhibitors could radically change the landscape of Alzheimer’s disease management, as they might restore normal microglial function and prevent further cognitive decline. The complexity of Alzheimer’s pathology demands innovative solutions like TIM-3 therapy that offer multi-faceted benefits, addressing both the clearance of pathology and supporting the neuroimmune function crucial for brain health.
Potential Outcomes of TIM-3 Based Treatments
If TIM-3 therapy proves effective in humans, it could mark a watershed moment in Alzheimer’s disease treatment. Rather than only managing symptoms, this innovative approach aims to alleviate the fundamental problems of plaque accumulation and cognitive decline. By repurposing already existing anti-TIM-3 antibodies, researchers can reduce development time and costs associated with creating entirely new drugs. As safety profiles for these antibodies are already established owing to cancer treatment applications, this strategy holds promise for smoother transitions into clinical use and more rapid deployment in the Alzheimer’s treatment landscape.
Moreover, the potential cognitive improvements associated with TIM-3 therapies could profoundly impact the lives of patients and caregivers. Enhanced memory function, better navigation skills, and an overall reduction in Alzheimer’s symptoms may reclaim a sense of independence and quality of life for many individuals affected by the disease. The hope is that through this targeted therapy, we could witness not just incremental benefits, but substantial enhancements in cognition and daily living.
Ongoing research into TIM-3 therapies must remain focused on elucidating the safest and most efficient methods for application in human subjects. As we gather more data from ongoing studies, there is optimism about the regulatory approvals of TIM-3-based interventions, possibly in the near future. The timeliness of this advancement is critical as the global demographic shifts continue to project a rising prevalence of Alzheimer’s disease. Targeting TIM-3 could pave the way for new treatment paradigms that not only promise better outcomes but also invigorate the scientific community’s resolve to tackle Alzheimer’s disease head-on.
The Future of Alzheimer’s Research with TIM-3
The exploration of TIM-3 in the context of Alzheimer’s disease symbolizes a forward leap in the field of neurodegeneration research. The findings that TIM-3 plays a central role in inhibiting microglial function could redefine therapeutic approaches, prompting a multidisciplinary effort to accomplish what has been elusive in earlier Alzheimer’s studies. Researchers are poised to pursue a comprehensive understanding of TIM-3 signaling pathways while collaborating across disciplines—from immunology to neurology—to generate a holistic view of the mechanisms at play in Alzheimer’s pathology.
This collaborative spirit may lead to groundbreaking discoveries not only involving TIM-3 but also in broader immunotherapeutic strategies against Alzheimer’s disease. As knowledge about how immune checkpoints manage neuroinflammation grows, it could inspire novel interventions that significantly alter the trajectory of not only Alzheimer’s disease but other neurodegenerative conditions as well.
As research progresses, the implications for public health and clinical practice remain vast. If TIM-3 therapies can secure robust cognitive improvements in patients, scaling these interventions could transform not just treatment methodologies but also the socio-economic landscape of care for aging populations. With Alzheimer’s predicted to impact millions globally, innovating around TIM-3 could catalyze change in how clinicians approach neurodegenerative diseases, ensuring that patients receive more promising, effective medications that target their disease at its root. As the scientific community continues to venture into uncharted territories within Alzheimer’s research, the prospects of improving quality of life for individuals facing this disease only grow brighter.
Implications of Findings on Alzheimer’s Community
The clinical implications of findings surrounding TIM-3 therapy extend beyond just the laboratory; they resonate through the entire Alzheimer’s community, encompassing patients, caregivers, and healthcare providers. Potential TEM-3 therapies may offer a much-needed beacon of hope amid a landscape where traditional treatments have failed to produce significant cognitive enhancement. Should such therapies advance to the clinical trial stage and yield positive results, it could galvanize an entire wave of new research, invigorating funding opportunities and the development of additional innovative treatment regimens.
For patients suffering from Alzheimer’s disease, the promise of alternative therapies that engage the immune system to clear harmful plaques brings renewed hope for better management of their symptoms. The understanding that there may be disease-modifying treatments on the horizon could significantly improve the morale of caregivers and families, who often bear the weight of the challenges posed by Alzheimer’s disease.
Furthermore, aligning funding and research efforts toward TIM-3 therapies could diversify treatment avenues available for Alzheimer’s disease and inspire researchers to explore related pathways. The shift could mark a departure from exclusively amyloid-beta-centric approaches to a more balanced viewpoint that considers immune-mediated strategies. This evolution in research focus might unlock new revelations about Alzheimer’s disease pathogenesis and lead to multifaceted treatment strategies that actually alter disease progression. Ultimately, as insights regarding TIM-3 therapies mature, they may redefine patient care and pave the way to collaborative efforts targeting neurodegenerative diseases.
Next Steps in TIM-3 Research
Looking ahead, the trajectory of TIM-3 research in Alzheimer’s disease is poised for significant development. The foundational studies indicating the role of TIM-3 in microglial inhibition set the stage for more advanced trials targeting this checkpoint. Next steps involve not only validating the initial findings in larger, more diverse mouse models but also beginning the transition to human clinical trials. Pioneering research efforts focused on TIM-3 will seek to determine the most efficacious methods for intervention, whether through monoclonal antibodies or small molecular inhibitors, and how these approaches can be safely integrated into existing Alzheimer’s protocols.
Researchers will also need to address potential challenges, such as ensuring that therapies targeting TIM-3 do not inadvertently disrupt beneficial immune functions or promote adverse effects. Rigorous preclinical trials will be essential to ascertain the therapeutic window of TIM-3 interventions and characterize the overall safety profile to ensure that patient welfare remains paramount throughout development.
In light of the recent advancements, the collaborative nature of this research will be crucial. As interdisciplinary teams gather to explore the intricacies of neuroimmune interactions, the journey from bench to bedside will ideally be streamlined through shared insights and pooled resources. The engaging dialogue among immunologists, neurologists, and pharmacologists could expedite the development of TIM-3-targeted therapies, uniting their efforts to forge more effective treatment pathways. As this challenging yet promising endeavor unfolds, the commitment to addressing Alzheimer’s disease through such innovative approaches will drive the scientific community towards discoveries that could reshape outcomes for a generation of patients.
Frequently Asked Questions
What is TIM-3 therapy for Alzheimer’s disease and how does it work?
TIM-3 therapy for Alzheimer’s disease involves targeting the TIM-3 checkpoint molecule, which inhibits brain immune cells known as microglia from clearing harmful amyloid plaques. By blocking TIM-3’s inhibitory function, microglia can become activated to reduce plaque burden, potentially leading to cognitive improvement in patients.
How does TIM-3 affect microglia function in Alzheimer’s disease treatment?
In Alzheimer’s disease, the expression of TIM-3 on microglia increases, preventing these immune cells from phagocytosing amyloid plaques. TIM-3 therapy facilitates the activation of microglia, allowing them to clear plaques, restore microglial function, and enhance cognitive capabilities.
What role do brain immune cells play in Alzheimer’s and TIM-3 therapy?
Brain immune cells, particularly microglia, play a crucial role in maintaining brain health by clearing plaques associated with Alzheimer’s disease. TIM-3 therapy aims to inhibit TIM-3 expression on microglia, promoting their plaque-clearing ability and improving cognitive function.
How can TIM-3 therapy lead to cognitive improvement in Alzheimer’s patients?
By removing the inhibitory effects of TIM-3 on microglia, TIM-3 therapy enhances the ability of these brain immune cells to clear amyloid plaques from the brain. This reduction in plaque burden can lead to significant cognitive improvement in Alzheimer’s patients.
What are the current research advancements regarding TIM-3 therapy for Alzheimer’s disease?
Recent studies have shown that deleting the TIM-3 gene in mice models of late-onset Alzheimer’s leads to enhanced clearance of plaques and improved cognitive behavior. Researchers are now investigating the potential of anti-TIM-3 antibodies in human trials to further this progress.
Why is TIM-3 considered a potential genetic risk factor for Alzheimer’s disease?
TIM-3 is linked to late-onset Alzheimer’s disease through genome-wide association studies, demonstrating that its polymorphism may significantly influence the likelihood of developing Alzheimer’s. This connection underlines TIM-3’s importance in both disease mechanisms and as a therapeutic target.
What challenges does TIM-3 therapy face in the treatment of Alzheimer’s disease?
One challenge for TIM-3 therapy is ensuring that anti-TIM-3 antibodies reach the brain effectively without causing vascular damage. The goal is to repurpose existing anti-TIM-3 antibodies to specifically target the pathophysiology of Alzheimer’s without adverse effects.
What implications do TIM-3 therapies have for future Alzheimer’s disease treatments?
TIM-3 therapies have the potential to shift Alzheimer’s treatment strategies by utilizing immune modulation to enhance plaque clearance, offering a novel approach compared to traditional methods that have shown limited success. This could pave the way for more effective Alzheimer’s disease treatments.
| Key Point | Description |
|---|---|
| Overview of TIM-3 Therapy for Alzheimer’s | The TIM-3 therapy aims to repurpose a strategy used in cancer treatment to enhance the clearance of amyloid plaques in the brain and restore cognitive function in Alzheimer’s patients. |
| Role of TIM-3 in the Immune System | TIM-3 is an inhibitory checkpoint molecule that prevents the immune system from becoming overactive. In Alzheimer’s, its high expression on microglia inhibits their function to clear harmful plaques. |
| Findings from the Study | Deleting TIM-3 in lab mice led to increased clearance of plaques, improved cognition, and changes in plaque behavior, suggesting potential therapeutic benefits. |
| Therapeutic Approach | The therapy may involve the use of anti-TIM-3 antibodies to block its inhibitory effects, enhancing microglial activity to clear amyloid plaques effectively. |
| Future Directions | Further research is ongoing to evaluate TIM-3 therapies in humanized mouse models, focusing on their ability to halt amyloid plaque formation. |
Summary
TIM-3 therapy for Alzheimer’s shows promising potential as a groundbreaking approach in the fight against this debilitating disease. By leveraging the immunological mechanisms that have proven effective in cancer treatments, TIM-3 therapy aims to enhance the brain’s ability to clear toxic amyloid plaques. Initial studies suggest significant cognitive benefits in animal models, indicating that this innovative therapy could pivotally alter the course of Alzheimer’s treatment, marking a hopeful advance for millions affected by the disease.